 |
Toxoplasma gondii is a protozoan parasite that infects most species of warm-blooded animals, including humans, and many mammals. T. gondii often do not develop symptoms in healthy individuals because of their immune system. However, the parasites remain in the body in an inactive state, and they can be reactivated in a person with weakened immune systems. For example, persons who acquire HIV infection causes toxoplasmosis accompanied with symptoms including fever, confusion, headache, seizures, nausea, and poor coordination. When pregnant woman becomes newly infected with Toxoplasma, the parasites are transmitted to the fetus, resulting in congenital toxoplasmosis (mental retardation, seizures, blindness, and death). The development of a Th1-biased immune response associated with production of IL-12, IFN-γ, nitric oxide and classical activation of macrophages is thought to be critical for effective control and eradication of T. gondii.
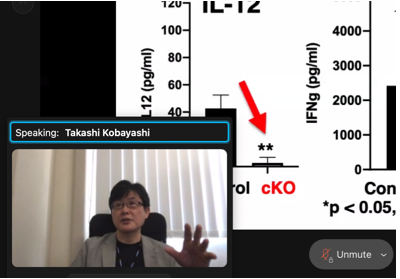
We have been investigating the molecular and cellular mechanisms controlling T. gondii infection using genetically modified mouse models such as astrocyte-specific STAT1 deficient mice and dendritic cell-specific TRAF6 deficient mice. STAT1 is involved in the transcriptional regulation of IFNγ-inducible genes in many types of cells, and TRAF6 mediates toll-like receptor signaling and induces IL-12 production in dendritic cells. We address the physiological relevance of STAT1 in astrocytes as well as TRAF6 in dendritic cells in T. gondii infection. In the symposium, I would like to share our recent data and discuss future perspectives for understanding and controlling the disease. |